

First I tell you what the textbook dogma is, and then how differently I imagine the phenomenon.
The widely accepted dogma
receptors in the plasma membrane are monomeric and inactive in the absence of stimulation. Ligand binding induces receptor dimerization leading to transmembrane signaling.
My view
A couple of my papers supporting the aforementioned assumptions:
Receptor-oriented drugs can be divided into two groups: low molecular weight tyrosine kinase inhibitors and monoclonal antibodies. Both of them are available against ErbB2, the member of the ErbB family which is often overexpressed in breast cancer leading to poor prognosis. Antibodies against growth factor receptors can grossly exert their action by one of the two mechanisms below:
Trastuzumab (Herceptin®) is a humanized monoclonal antibody against ErbB2. It exerts its action by altering/inhibiting the signaling mediated by ErbB2 (mechanism 1), but it is also known to cause immune system-mediated attack against the cancer (mechanism 2). Unfortunately, the development of resistance against trastuzumab is practically inevitable. Several assumptions have been put forward to explain the development of resistance (summarized in the following papers:
We identified epitope masking as a mechanism leading to trastuzumab resistance. The expression of the large cell surface mucin MUC4 was shown to inhibit the binding of the antibody to ErbB2-overexpressing cells.We showed that pericellular hyaluronan also blocks trastuzumab binding and inhibition of hyaluronan synthesis increased the efficiency of trastuzumab treatment in experimental animals. We extended these investigations to clinical samples of ErbB2-overexpressing patients and showed that binding of trastuzumab to ErbB2 was inhibited by a high pericellular density of hyaluronan. Hyaluronidase treatment of the samples significantly increased trastuzumab binding.
Our hypothesis: Trastuzumab resistance is caused by a variety of factors. Among these, epitope masking seems to be important. MUC4 and hyaluronan can sterically block the access of antibodies (and effector cells) to the tumor. Alternatively, (especially hyaluronan) is expected to increase the interstitial pressure around tumor cells inhibiting the transport of molecules from blood vessels to the tumor. Our results imply that inhibition of hyarluronan synthesis or induction of its breakdown can significantly increase the potency of trastuzumab, and potentially other drugs, in the treatment of breast cancer.
My papers supporting the claim above:
Quantitative ratiometric FRET measurements in microscopy:Although
methods abound for the measurement of FRET, ratiometric measurements,
i.e. the determination of the fluorescence intensities in the donor,
FRET and acceptor channels, has its appeal, e.g. time course
measurements are possible. We have developed a method which takes
spectral overspills and the alpha factor (explained under the next
point) into account to yield pixel-by-pixel FRET values.
The paper:
Intensity-based energy transfer measurements in digital imaging microscopy. Eur. Biophys. J. 27: 377-389 (1998)
Calibration of FRET values in flow cytometric measurements
using fluorescent proteins:
In FRET an excited donor molecule is quenched by a nearby
acceptor, i.e. an excited acceptor is "created" from an excited
donor. Since the donor and acceptor fluorescence intensities are measured by
different detectors, their fluorescence intensities aren't comparable, i.e. the
intensity of one donor molecule in the donor channel isn't identical to the
intensity of an acceptor in the acceptor (or FRET) channel. The ratio of the
fluorescence intensity of the same number of acceptor and
donor molecules is called the alpha factor. We have worked out a way to
determine this parameter in flow cytometric measurements of FRET using
fluorescent proteins.
The paper:
Novel calibration method for flow cytometric fluorescence resonance energy transfer measurements between visible fluorescent proteins. Cytometry, 67A: 86-96 (2005)
Quantitative determination of the size of protein homoclusters using flow cytometric homo-FRET measurements
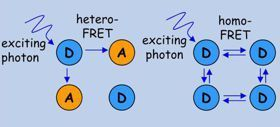
FRET has two major types:
1. conventional hetero-FRET in which spectroscopically different molecules interact;
2. homo-FRET
in which the donor and the acceptor are molecules of the same species.
In homo-FRET the excitation energy of the first donor is spread all
across the cluster if the fluorophores are within FRET distance from
each other.

Homo-FRET is only manifested in the decrease in the fluorescence anisotropy of the donor. Although the donor population is excited by polarized light, the donor molecules secondarily excited by homo-FRET will be almost randomly oriented relative to the primarily excited donor, therefore the mean fluorescence anisotropy of the population will be lower than in the absence of homo-FRET. Conclusion: the lower the anisotropy is, the more homo-FRET takes place.

The problem is that in addition to homo-FRET other factors also influence fluorescence anisotropy, therefore the relationship between anisotropy and homo-FRET is ambiguous. Our method: cells are labeled with a mixture of labeled and unlabeled antibodies, and the fraction of the labeled antibody is changed from 0% to 100%. If the fraction of the labeled antibody is low, the probability that there will be two or more labeled antibodies in the cluster (and therefore homo-FRET can take place) is low. By increasing the fraction of the labeled antibodies, the probability of homo-FRET increases.
Quantitative measurement of the stoichiometry of protein heteroclusters using FRET-sensitized acceptor bleaching (FSAB)